Managing deliverables through the product development process
Imagine a quick and easy way to replace the legacy Excel checklists with a guided process from user needs definition to approval documentation. How beneficial would that be to have the complete workload, responsibilities, timelines considered and created by one click? Read on to learn how.
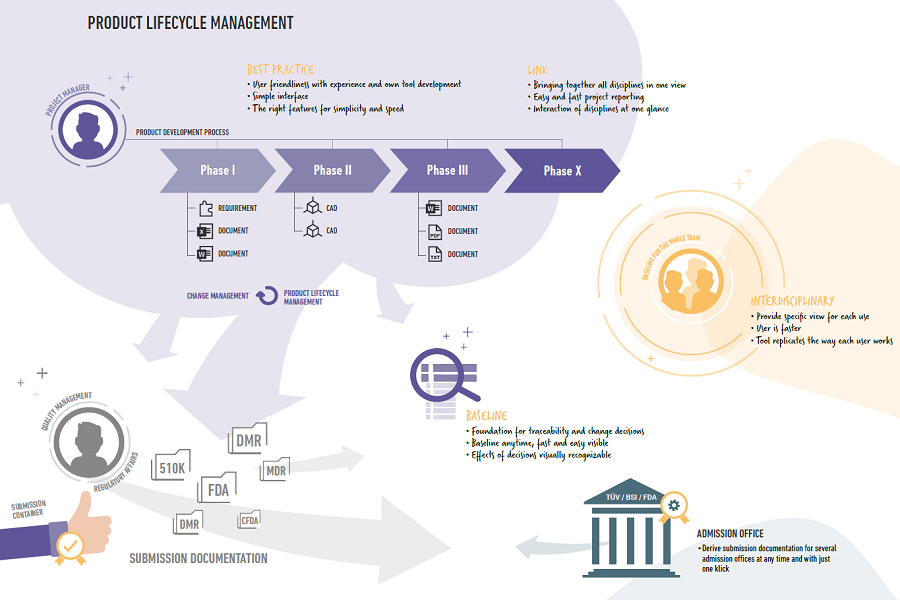
Guided product development process
Often project managers spend a lot of time on non-productive tasks such as defining the correct scope, bringing each team member up to speed, connecting the right people, triggering post-actions, etc. One way medical device project managers address these issues is using the Teamcenter Medical Device Solution that Siemens Digital Industries Software solution partner, avasis, created.
The solution supports compliance with processes and facilitates submission management, increasing efficiency, accelerating innovation in development, reducing costs, and ensuring compliance with regulatory guidelines and quality. Since the project, with all its deliverables, can be created from a template the scope definition needs only to consider the expectation. The idea is to combine all the disciplines (requirements, software, mechanical engineering, return authorization, quality management, etc.) connected into one view, making the collaboration much easier and the project scope and progress much clearer at any time to each team member. It also facilitates capturing dependencies between the disciplines and enables seamless communication.
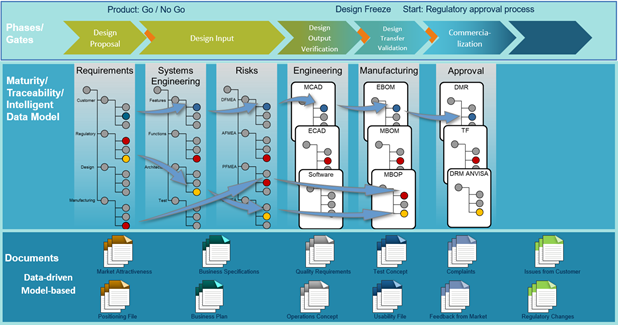
Accelerated submission file creation
We talked to several customers and saw that the process for creating the submission file for a specific product was a separate process from the product development. The certification of the product started too late and valuable time was lost.
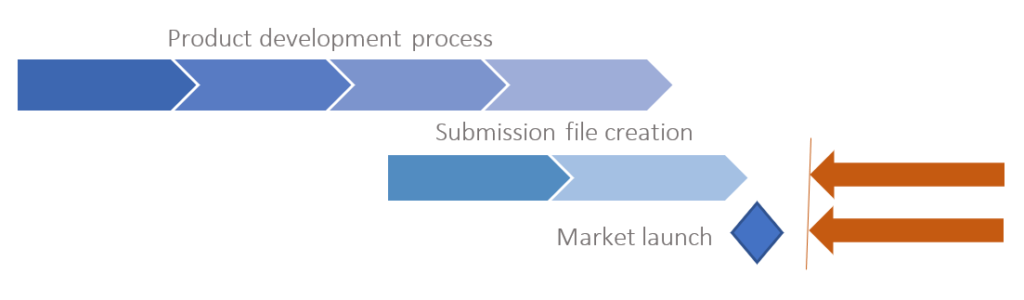
The submission file creation is supported by
- digital trace links
- the possibility to start the creation in parallel to the product development process
- utilization of just one source for all relevant information
These points facilitate the certification process to be more reliable, easier and faster.
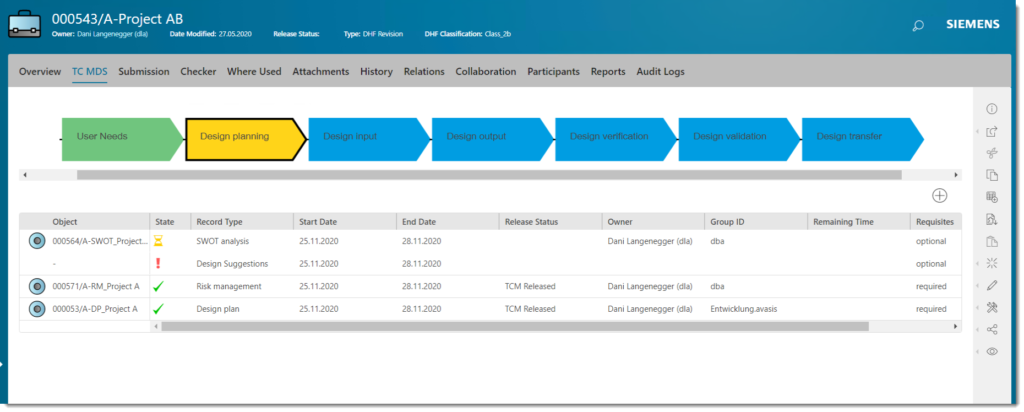
Product development process 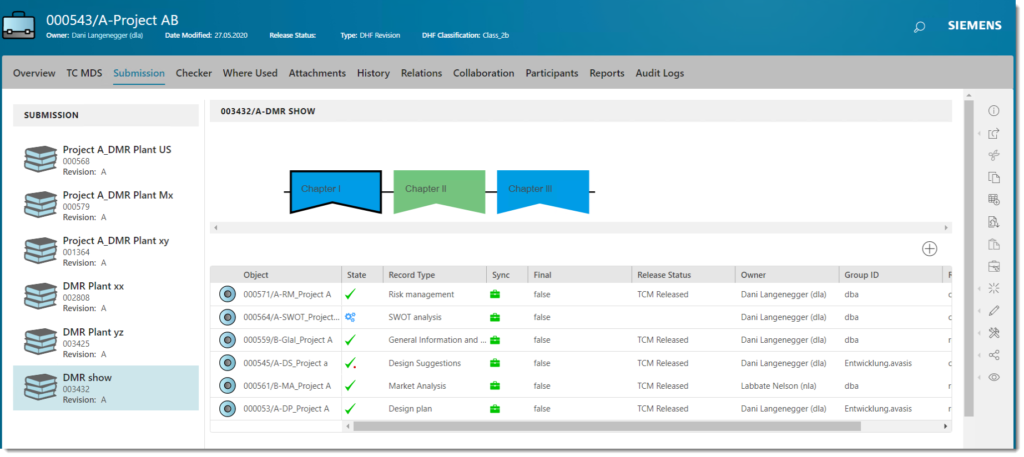
Individual view (e.g. Submission file)
Finally, the solution allows us to create an interdisciplinary view that replicates the effective need. That is, it provides specific views for each role, reflecting the way each user works, while increasing productivity. The development and submission creation process are independent and a baseline for each submission can be created at any time, providing synchronization status at all times. Baselining is important because it provides the foundation for easy traceability and decision making for changes. The effects of decisions can be visually displayed at any time, quickly and easily.
Furthermore, the solution displays straightforward status symbols and color-coding, so it is well understood by people who are not working with the tool daily. This helps to ensure the solution is well-accepted and benefits everybody. Read more about Teamcenter.
Daniel Langenegger is the Product Manager for Teamcenter Medical Device Solution at avasis, a Siemens Digital Industries Software Smart Expert Solution partner. He supports project managers, regulatory affairs, quality managers, and engineers to fulfill guidelines and optimize their respective processes.


