Design Excellence in Medical Device: Design File Integrity

In this post we will explore Design File Integrity – accessible, integrated, high quality data throughout the product lifecycle for trusted compliance and product maintenance – as part of our Design Excellence series for Medical Devices. Follow along to learn how 5 key areas of Design Excellence combine multi-disciplinary design collaboration with advanced design tools and multi-physics simulations to achieve competitively differentiated, premium-value devices.
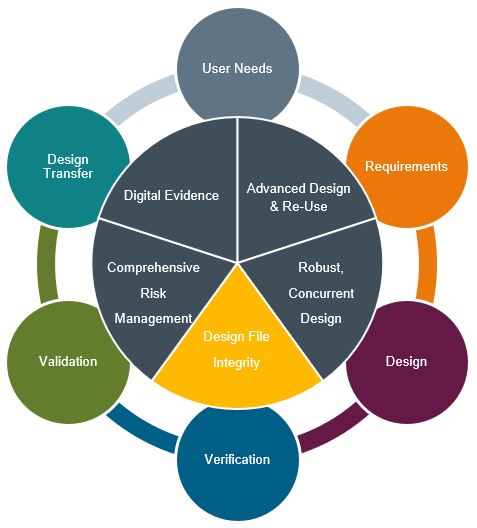
Design File Integrity
Design files are compound structures, entangled with one another, multi-authored, multi-domain, and ever changing. Yes, they are regulated and in many medical device companies they are viewed by management or QA/RA only as a compliance challenge. However, those who really understand know that design files are the foundation of the organization – the core intellectual property – and must be treated with utmost care and respect. The value of robust design files will save the organization time, money, and preserve competitive advantage. Compliance simply follows. When discussing design file integrity, however, this implies trust. Do you trust your design files?
This question of trust is best answered through observed behaviors in your organization:
- Do you struggle to understand intent or linkages in your design file data?
- Is there a high dependency on a certain individual or individuals to interpret what was intended or play the role of historian?
- Is there a stream of DHF remediation projects and hiring/contracting for this activity?
- Does your sustaining engineering team struggle to plan and execute effectively due to missing or unclear product information?
- Are there Change control struggles with the span & scope of change planning as well as difficulty accessing affected objects?
- Do you trust your design data & supporting documentation enough to re-use content in new designs or versions/configuration instead of creating from scratch?
- Have you experience supplier issues such as version control, correct design documentation, component qualification issues/delays due to missing, incomplete, or ambiguous information?
- When performing design transfer are you confident your production & process controls match key performance and risk characteristics?
- Have you experience audit compliance issues, CAPAs, investigations related to design control data?
- Are there shadow IT groups operating within your engineering teams?
If you answered yes to one or more of these questions you may have issues with design file integrity.
Effectively creating and managing the Design Files [Design History File (DHF) or Technical File and the Device Master Record (DMR)] requires one source of truth for the organization. This system of record must be accessible to all required parties and automate the complexity of workflows and traceability across design inputs and outputs. It must be able to handle multi-BOM management (Mechanical, Electrical, Software) as well as ensure integrity of interactions with suppliers and production.
Furthermore, a solution must be useable in both experience and utility! If the user experience is poor or inadequate, adoption will languish. Poor utility may lead to divided solutions or design file integrity failure. Either case may lead to uncontrolled shadow IT solutions or even mixed paper/digital repository hybrid solutions.
As products have increasingly become cross discipline, so has the need for tightly coupled application and lifecycle management environments in today’s leading medical device companies. This approach ensures design file integrity while maintaining the purpose-built capabilities required for each engineering discipline to succeed. Additionally, this environment establishes that single source of truth & master control for all your design authoring applications. BOM’s, configuration management, trace matrices, structure, history, change control, etc. of all design objects become second nature and automatically managed. In addition to controlling core design data such as CAD, specifications, schematics, & software; leading companies manage their product design related information such as sourcing & supplier integration, simulation management, and material compliance & sustainability.
Medical device design file complexity is only going to grow – and once the integrity of your design files is lost it is very difficult and painful to restore. It is clear that modern design excellence requires a comprehensive digital environment to tackle this challenge.
To Learn more about Design Excellence at Siemens please visit here.