Webinar: How to accelerate innovation with PLM for Medical Devices

The need for digitalization in the industry
Many engineers face similar challenges and pressures in the industry, even without a product lifecycle management (PLM) system. The biggest challenge they face is the need to move faster. However, it is hard to bring devices to market faster when designing and developing these devices is more complex than ever. These two factors are at odds with one another.
With smarter devices, there are now more electronics and software that goes into making a product and its functionality, which means more collaboration is needed. Smarter and smaller devices are more intricate and take even more attention to detail and risk than before. And as always, there are also the ever-present regulatory requirements that must be met and complied with when it comes to developing medical devices. This process can be burdensome.
In addition to these challenges, engineers have insufficient integration between domains, including labeling, software, mechanical and more. Many organizations still use manual methods in the PLM process. There seem to be complications in the handoffs and interchanges between project teams or individuals. This lack of automation and digitalization can lead to data incompatibilities and translation.
Accelerated collaboration with PLM
The new Software-as-a-Service (SaaS) PLM for Medical Devices solution addresses the pressures that engineers face on a daily basis and provides an approach to digitalization. This can help to accelerate innovation in medical device processes.
PLM for Medical Devices is based on three principles:
- Design Data Management – This is where a digital twin can be created to replicate a product and collect all the design inputs in a more data-rich model-based way that provides more traceability.
- Product Line Management – This gives teams the ability to manage the design history, submissions to regulatory bodies and device master records of a product in one place.
- Quality Process Management – This is an effective way to trace a product’s risks, requirements and V&V testing, while also dealing with change control and CAPA processes.
A simplified solution framework
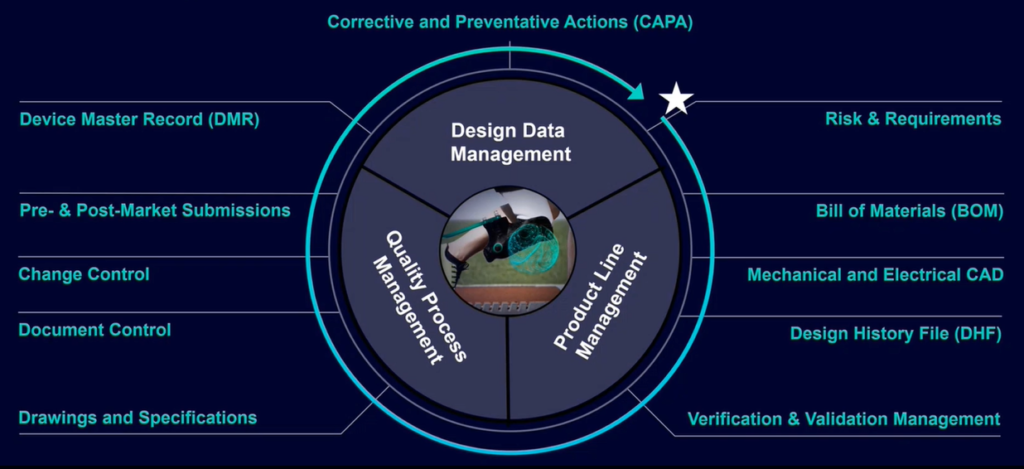
PLM for Medical Devices has a simplified framework to make using the solution easier. The framework has many abilities and features, including:
- An intuitive user interface that includes intelligent dashboards and discussions
- The ability to quickly find information, such as BOM, design data, V&V testing and more
- Built-in risk analysis according to ISO 14971
- Synchronized product and manufacturing COM configurations
- An integrated, multi-CAD design environment
- Automated design history file management
To learn more, watch the free, on-demand webinar to accelerate your team’s innovation and collaboration with our instant-on, SaaS PLM for Medical Devices solution here.
To read more blogs about the medical device and pharmaceutical industry, go here.