Streamline Medical Device Development with PLM – Available Now!

Medical device manufacturers of all sizes now have a cost-effective way to tackle complexity and regulatory demands with faster time to market. Siemens PLM for Medical Devices is an instant-on, cloud-based product lifecycle management (PLM) system that helps maintain data integrity and provides the infrastructure to ensure compliance for medical device development.
Fragmented IT systems. Siloed Excel sheets. Engineering workarounds that violate compliance. All these inefficiencies can be a thing of the past with cross-domain collaboration and digitalization.
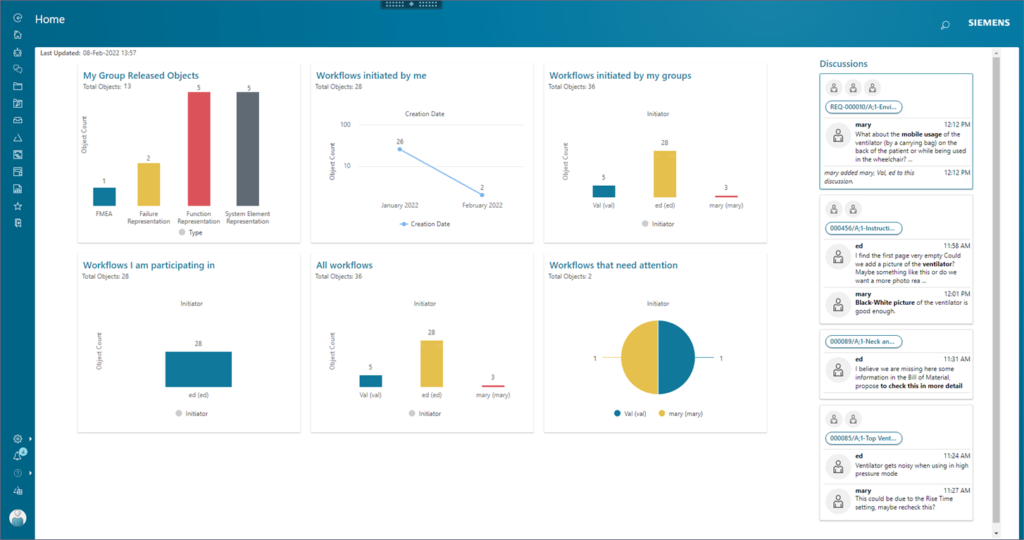
Benefits of PLM for Medical Device Development
When mechanical, electronic and software engineers work together, global data stay synchronized and device design information is more easily shared with manufacturing. Automating, standardizing and optimizing processes leads directly to cost savings and speedier development.
PLM for Medical Devices enables re-use of development data and evidence to better control complexity and maintain reliability. And that helps increase your competitive advantage.
Some of the benefits include:
- Designs delivered faster, with higher quality, while ensuring regional regulatory compliance
- More accurate and flexible engineering
- Improved product quality and data integrity
- Reduced legal and clinical risks
- Ability to act quickly by moving away from paper-based change processes
- Capability to easily share design information across teams
- SaaS delivery for anytime, anywhere access
Accelerate medical device development through digitalization
PLM for Medical Devices incorporates three key areas: design data management, product line management and quality process management.
Our solution supports your end-to-end business processes with a preconfigured offering that will grow with you. Design teams can collaborate and share work in progress (WIP) to define and complete the development processes. It includes built-in best practices such as Design History File, risk analysis and requirements, CAPA and process management. Connection to ERP and manufacturing systems enables compliance with various regional regulations.
Features include:
- Built-in risk analysis set to ISO 149 standards
- Multi-BOM management for integrity of interactions with suppliers and production
- An integrated, multi-CAD design environment
- Complete traceability of DHF/DMR/submissions for simplified regulatory interactions
- Live reporting on medical device verification and validation (V&V) testing
- Object-based document management for ease of design transfer
- Revision and version control of regulatory controlled documents
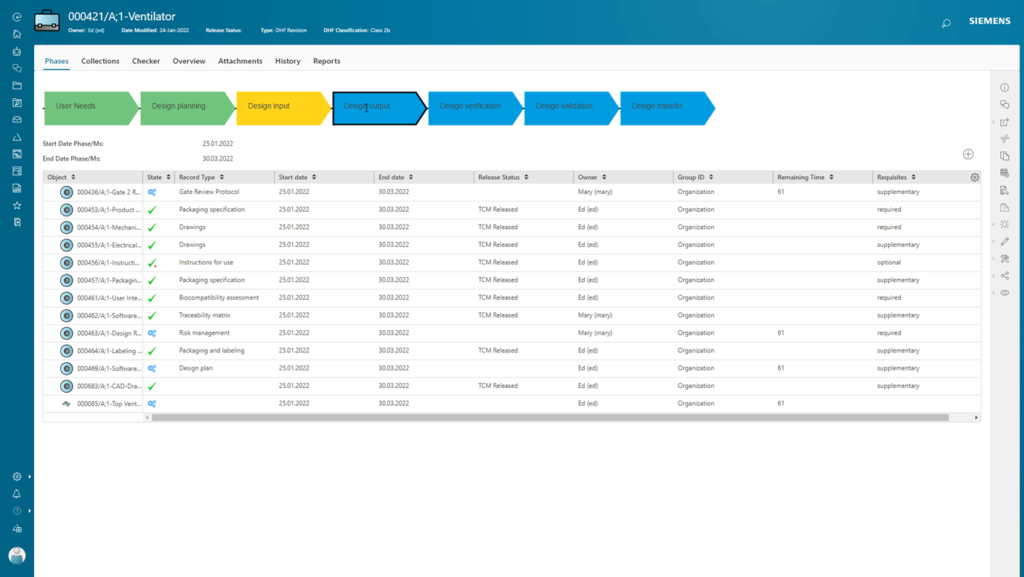
Streamline your medical device product development
Deliver innovation at warp speed with PLM for Medical Devices. Implement a truly connected infrastructure that fast-tracks domain-specific progress with robust design tools. The solution is already proving itself with some of the largest medical device manufacturers. How can we help improve your efficiency?
Learn more about PLM for Medical Devices and discover how your company can reduce product development costs and compliance issues. A free 30-day trial is available so you can try for yourself!


