Chocolate, mayo and the science of slippery stuff: Predict viscosity of complex fluids

Who doesn’t remember Sir G. I. Taylor, that brilliant 20th-century British scientist whose work shaped our understanding of fluid dynamics? I still recall my university lab sessions, where, using classic instruments (after all, why change something that’s worked for decades?!), we’d reproduce some of his fascinating observations.
Viscosity – the most underestimated fluid property
My favorite? The Taylor-Couette flow experiment! If you choose your fluids just right, you can literally go back in time thanks to ultra-laminar reversible flow; it’s incredible!
At the heart of this stunning yet somewhat puzzling experiment is the equally mysterious property of fluid viscosity. And while viscosity plays a governing role in fluid mechanics, its fundamental physical origins lie buried deep in molecular interactions that lead to the internal friction “within” a fluid. As such a new player must come into play when trying to truly predict viscosity of complex fluids: Computational chemistry. And yet, as we shall see, reproducing such feats as the Taylor-Couette observation in computational chemistry is no small task. It’s not so much a question of system size, although that does limit the scope of our experiments, but rather the complexity of correctly solving the equations to predict the exact behavior. And this precision is crucial when predicting fluid viscosity.
Predicting viscosity of chocolate – sweet!
In Simcenter Culgi 2211, we introduced the ability to model systems under shear at the coarse-grained level, enabling an evaluation of their viscosity. This worked well for well-mixed models, such as triglycerides, which you can find in your favorite chocolate.
We were able to predict their experimental viscosities with good precision and accuracy, as you can see:
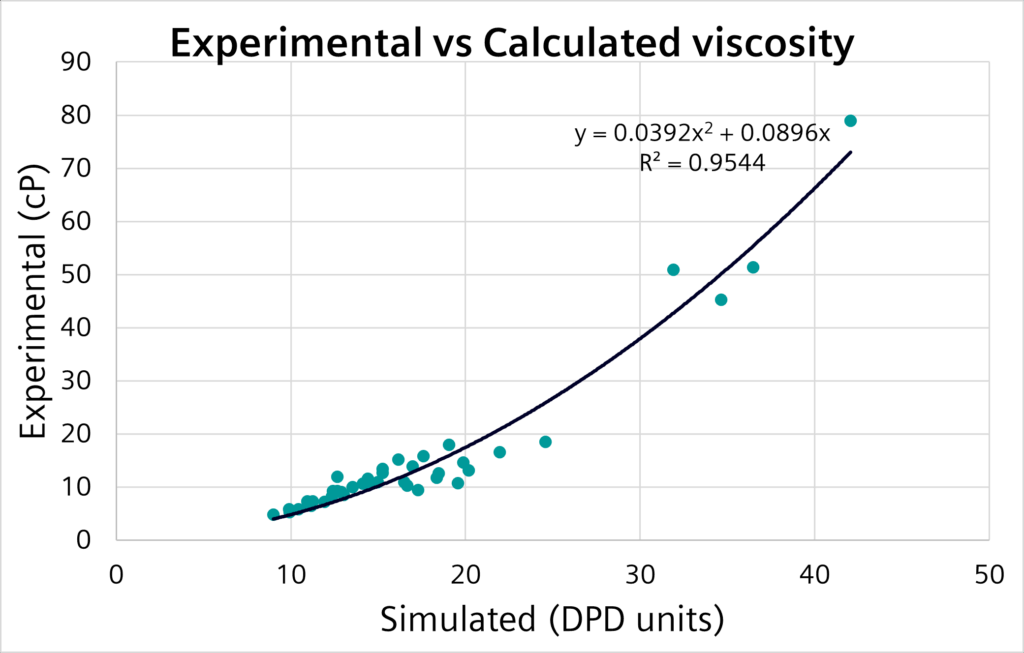
While this was a pretty sweet first step (after all we are talking chocolate here), we didn’t stop there! This was just the beginning in our quest to better predict viscosity of complex fluids. We wanted to conquer mayonnaise – which in terms of viscosity is on another level!
Stirred, not shaken – deformation of a droplet under shear
To get us there we needed to see if we could reproduce another experiment by Sir Taylor: In 1934, he investigated the impact of stirring processes that create emulsions (such as our mayonnaise). To put it simply, an emulsion is a mixture of two immiscible fluids, like oil and water. The key question Taylor tried to answer: what happens in an emulsion during mixing steps in production?
It is not that obvious that mayo is an emulsion – just believe me it is and for now imagine shaking a vinaigrette bottle: you emulsify the oil into tiny droplets within the vinegar. What about already existing droplets? How are they affected by this shaking, or shearing?
This is precisely the use case that Sir Taylor studied, creating an experimental setup to shear oil droplets in water and observing the impact of various factors, such as shear rate and oil viscosity. In molecular terms his experiment is about correctly predicting the deformation of a droplet under shear – and while doing so learn something about the viscosity of the system.
Make no mistake – trying to simulate this historic experiment is neither a throwback just-for-fun exercise nor a cooking class for nerds – it is a very recent relevant topic in many industries where mixing is key and emulsions are omnipresent, including Cosmetics, Pharmaceuticals and – you guessed it – Food & Beverages.
Predicting viscosity of complex fluids – failed
For such immiscible systems, as oil droplets in water, predicting viscosity is a different story. Our previous methodology, while powerful for homogeneous mixtures, couldn’t accurately represent these separate phases. As you can see in the video below the legacy method poses a zero shear singularity in the center of the emulsified drop leading to rather useless artifacts in the attempt to derive viscosity from the numerical experiment.
Predicting viscosity of simple fluids – check
We therefore stepped up our modelling game with a new methodology in our latest release, Simcenter Culgi 2511 that shall capture single phase and emulsified systems equally well. Hence, before checking whether we could replicate Taylor’s observations, we validated our new implementation with pure systems of small molecules. If you cannot even predict the viscosities of what we consider to be simple systems, you’d be in trouble. Right?
So, we decided to try our hand on C6 alkanes with small structural differences. They are well documented; thus, we would be able to check our implementation’s accuracy:
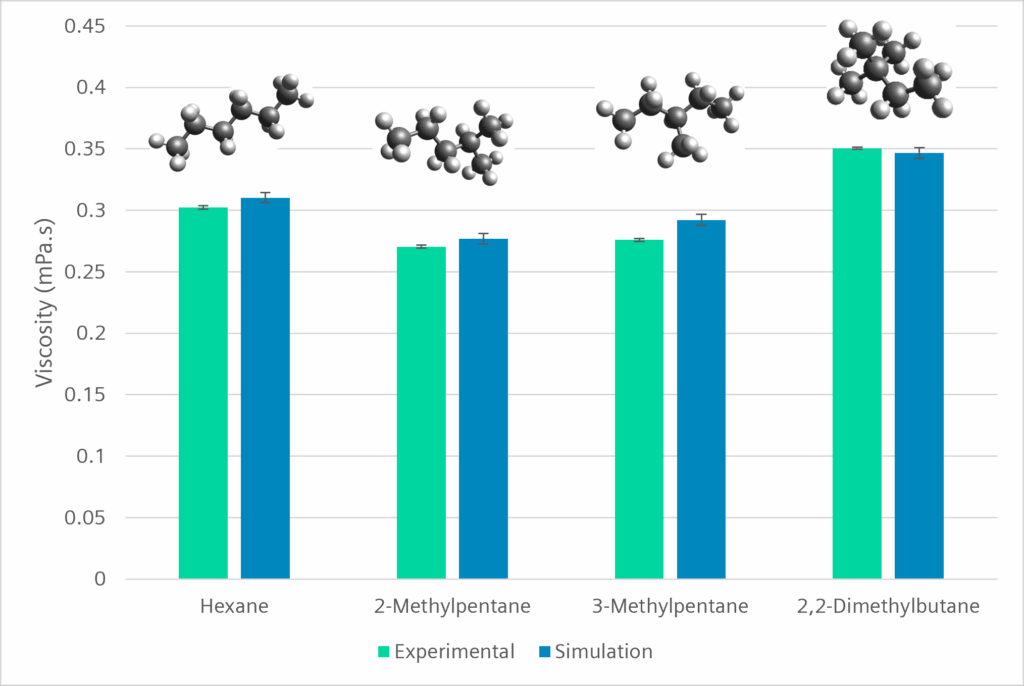
As you can see, we have excellent accuracy in predicting their viscosity, and more importantly, we are able to capture the impact of the molecule structure on the viscosity. The question remains, does this still hold when moving to more complex fluids.
Predicting viscosity of complex fluids – check
So, we went on to look at mayonnaise, aka the oil droplet in water. And -spoiler alert! – we have successfully modeled the impact of shear rate on the deformation of oil droplets in water at ambient temperature:






We are extremely proud to now offer, within Simcenter Culgi, a significantly more accurate and robust tool for the prediction of viscosity – of any complex fluid. You can now confidently model and predict the viscosity of formulations under shear, whether it’s chocolate, mayonnaise, polymer blends, or any other complex system at any pressure and temperature!
So the next time you have some fries with mayonnaise, a salad with a nice vinaigrette on the side, followed by a yummy chocolate cake, remember to tell your date about how to go back in time with Taylor, the mysterious nature of viscosity – and the power of Simcenter Culgi simulation to predict it.
Until then…