How to achieve continuous monitoring in medical device design control

Medical device design control is notoriously riddled with errors and inefficiency, but it doesn’t have to be. Continuous monitoring can help manufacturers gain better command over process complexity, including looped feedback, review steps, improvements and more.
Siemens helps tackle these common medical device design control hurdles with continuous monitoring that interconnects data for visibility across activities and establishes dedicated platforms for reuse of requirements, risk analysis and testing.
Read more about medical device process monitoring
A data-driven, holistic approach to design control process monitoring
Our medical device process monitoring tools come with an intuitive interface that follows the status of each project stage.
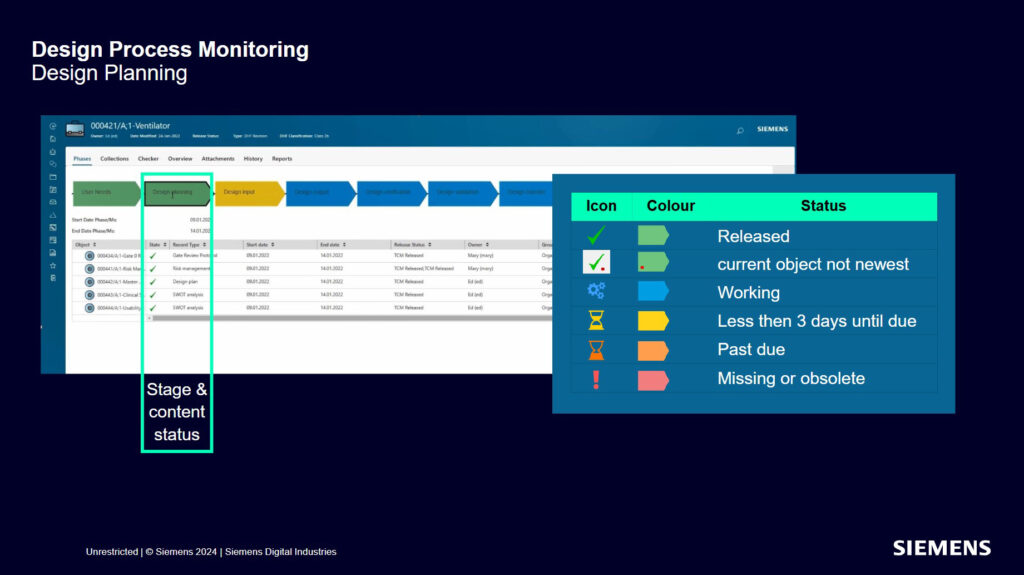
Color codes and icons enable you to quickly identify blocking points, monitor process advancement and anticipate actions.
Key continuous process monitoring capabilities:
- Track, review and approve changes
- Compare revisions directly
- Built-in electronic signatures
- Integration with Microsoft 365
Design changes. Use a basic workflow for change control, with primary details summarized on a single overview screen. All data is tracked and viewable for compliance with FDA 21 CFR part 11 regulations.
Design Review. Enable robust compliance auditing and insights. Easily trace data, testing and real-world results.
Benefits of Siemens Design Control for Medical Devices process monitoring
Improve efficiency and device delivery by optimizing the time spent on rework and corrective actions. Implementation helps reduce errors, improve communication and speed up the design process.
Custom configuration enables our solution to grow with you as design processes become more complex. Users can also:
- Monitor in real-time
- Gain insight into every step of the design process
- Easily view project statuses
- Easily share design information across teams
Learn more about Siemens Design Control for Medical Devices
Design control with Siemens PLM for Medical Devices
Siemens Product Lifecycle Management (PLM) for Medical Devices is our integrated end-to-end product development system that tackles complexity and regulation through re-use, context capture and collaboration. Manufacturers improve visibility and traceability while reducing costs and compliance risks to produce competitively differentiated, premium-value devices.
PLM for Medical Devices helps manufacturers overcome siloed processes with an integrated approach to quality-by-design. Increase agility with more flexible ways of working, including:
- Quality process management. Gain the ability to execute modular and discrete re-use of requirements, risk analysis and testing objects from similar designs.
- Design History File (DHF) management. New project setups are template-driven and configurable, so users have the ability to select the correct DHF type depending on market and risk class.
- Device Master Record (DMR) management. Digital design transfer enables easier, faster and more accurate control.
Read the Siemens PLM for Medical Devices e-book
Learn more about Siemens Design Control process monitoring for medical devices
Siemens Design Control for Medical Devices design process monitoring tools enable effective, compliant design files throughout development from the Design History File (DHF) to design transfer.
Download the process monitoring for medical devices e-book now.