Combining system simulation and 3D CFD for haemodynamic modeling
Generating digital evidence for medical devices
Simulation can be used in many applications in the life sciences industry, including electrical design, mechanical design, vibration, acoustics and more. Simulation is often used for medical device design, in which objective evidence is provided to prove a design meets certain requirements. The most important requirements include safety and efficacy. There are many traditional ways to prove a device is safe and efficacious, such as bench testing, animal testing, cadaver testing and clinical trials.
Simulation is long established as a method for generating proof that a medical device meets a requirement. But simulation can be used in other ways as well. When it comes to simulation, we are talking about digital evidence generation. Digital evidence is essentially evidence generated by computer modeling and simulation. “In-silico” trials create digital evidence using computer simulation to evaluate drugs and devices to augment clinical trials or other testing methods.
In the medical device industry or life science industry, simulation is used to create digital evidence in the following areas:
- For device design – predict the performance of the device before building it
- To generate evidence of device performance as part of a regulatory submission – reduce the time and cost of regulatory approval
- For diagnosis and/or intervention planning – predict the outcome of a medical procedure before operating
Simulation credibility is key
Simulation is not useful unless it is credible. There must be trust in the predictive capabilities of a computational model in the specific context of where it is being used. Once that credibility is established, organizations can use simulation all throughout the design lifecycle. From early concept building where costs are relatively low, simulation can help to reduce the number of prototypes needed and therefore the ability to get a product to market sooner.
Simulation software must have the ability to be credible based on the range, realism and physics models it uses. This is a very important metric. Other metrics include having the ability to perform different types of simulations, such as 1D modeling (also known as system simulation) or data management. In a larger sense, teams need a tool that can give them a sense of traceability from requirements through to engineering data. Ultimately, simulation should provide an organization with insight into design exploration, along with data analytics and visualization, to help identify key parameters.
Best practices for CFD haemodynamic modeling
About in-silico trials
As mentioned above, in-silico clinical trials are a way of proving design efficacy and safety. These are becoming more popular in the medical device industry. In-silico trials are individualized computer simulations used in the development or regulatory evaluation of medicinal products, devices or interventions. In general, traditional clinical trials can cost upwards of $10 million and take, on average, five years to complete. In-silico trials can reduce the number of patients, follow-ups and time to market that is needed.
Simulation phases
There are three phases of simulation:
- Pre-processing Phase – Specify boundary conditions, coupling to Simcenter AMESIM or other software, define any metrics not included in the software, and complete the meshing pipeline.
- Simulation Phase – Run each timestep and record results at specified intervals.
- Post-processing Phase – Generate contour plots of the flow fields, and extract information for subsequent interpretation.
Setting up boundary conditions
The majority of CFD haemodynamic studies use velocity inlet–pressure outlet boundary conditions. The inlets are prescribed to be the velocity or mass flow waveforms. In regard to outlets, there are a few options, including zero-pressure or “bleeding out” outlet, prescribed flow split percentages based on daughter vessel areas, or Windkessel models. Lastly, for walls, non-slip conditions versus fluid-structure interaction methods can be used.
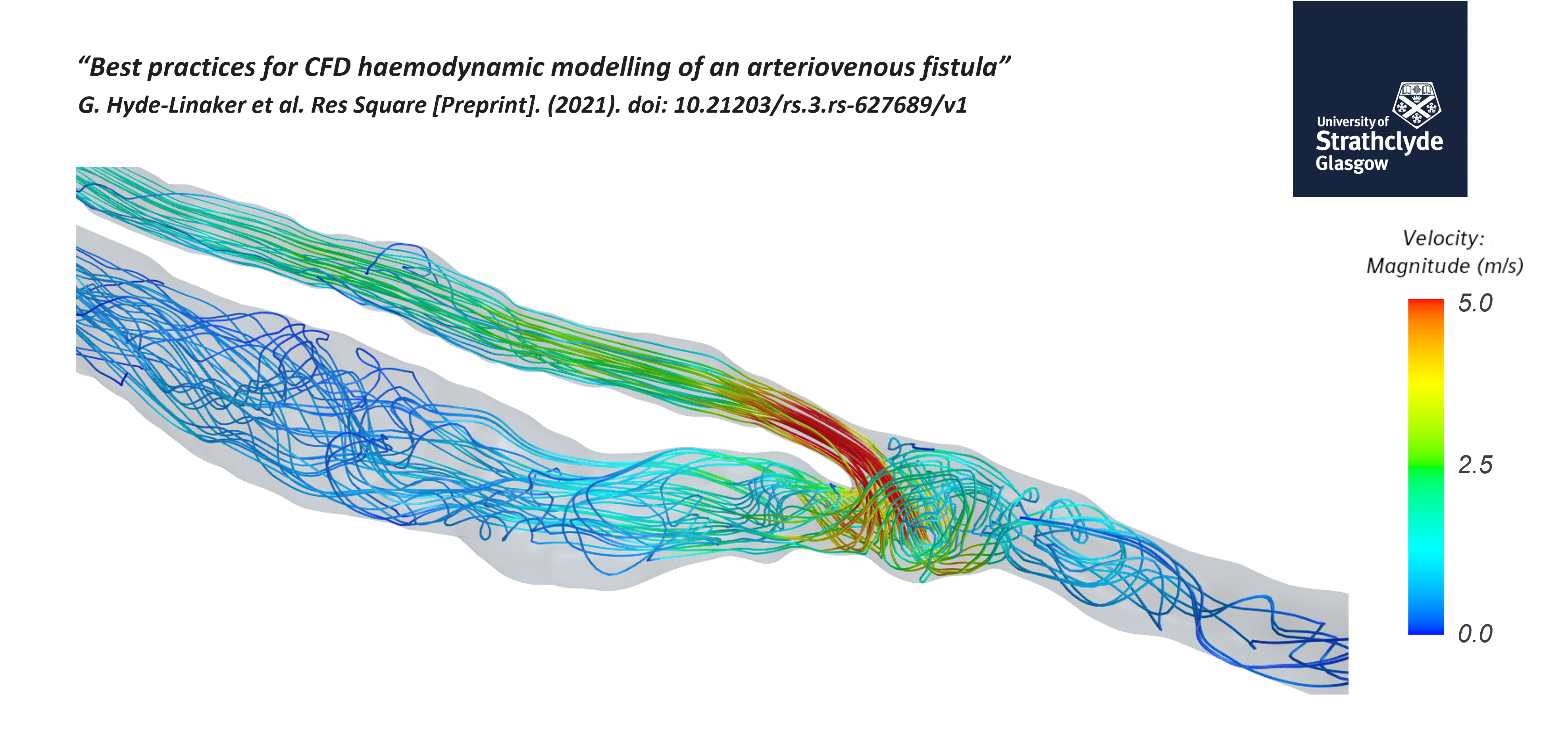
Patient-specific computational haemodynamics for a surgically created AVF
Clinical Background
End-stage renal disease (ESRD) patients require renal replacement therapy for survival. Without this transplantation, hemodialysis is needed to keep blood clean, which requires vascular access. The gold standard for vascular access is considered an arteriovenous fistula (AVF), which provides superior patency rates, lower infection rates and longevity. However, the majority of AVFs fail due to neointimal hyperplasia or other reasons.
When it comes to compiling studies, there are less than ideal patient-specific studies on ESRD patients. With Ferumoxytol-enhanced Magnetic Resonance Angiography (FeMRA), patient-specific datasets can be used for investigating proximal haemodynamics to the anastomosis (where the vein and artery connect) and for multi-patient cohort studies for establishing success predictors.
The objective of the study
In the webinar, the speaker dives into a paper about completing a simulation from the ascending aorta to the cephalic vein. The objective is to create a single computational domain and compare the haemodynamics before and after surgery.
Watch the webinar on simulation
To learn more, watch the free, on-demand webinar here.
You will learn about:
- Use cases, in which patient-specific treatments used simulation
- The impact of in-silico trials
- Workflow of FD haemodynamic studies
- How Simcenter STAR-CCM+ can be easily used for robust CFD studies
To learn more about the medical device and pharmaceutical industry, check out our blog network here.