What’s new in Opcenter Execution Pharma version 2205

Simplifying the Process Instruction program rollout
We are excited to announce that the latest version Opcenter™ Execution Pharma v. 2205 has been released!
Opcenter Execution Pharma (Opcenter EX PH) has moved to year- and month-based version numbering. Therefore, version number 2205 follows version number 6.2.4. Opcenter Execution Pharma version 2205 introduces several enhancements, increasing out-of-the-box features and simplifying the installation and footprint of the system.
Opcenter Execution Pharma, which is a part of the Xcelerator™ portfolio, the comprehensive and integrated portfolio of software and services from Siemens Digital Industries Software, enables all regulated processes to be managed without paper-based procedures or documents. The system offers easy configuration and an out-of-the-box (OOTB) functionality, which allows users to design any process without specific information technology (IT) skills.
Fully compliant with the U.S. Food and Drug Administration (FDA) and Good Manufacturing Practice (GMP) regulations, the system optimizes batch manufacturing processes and helps streamline resources, such as user guidance, equipment allocation and standard operating procedures. It also systematically controls execution at all stages, either human operations or operations controlled by the automation layer.
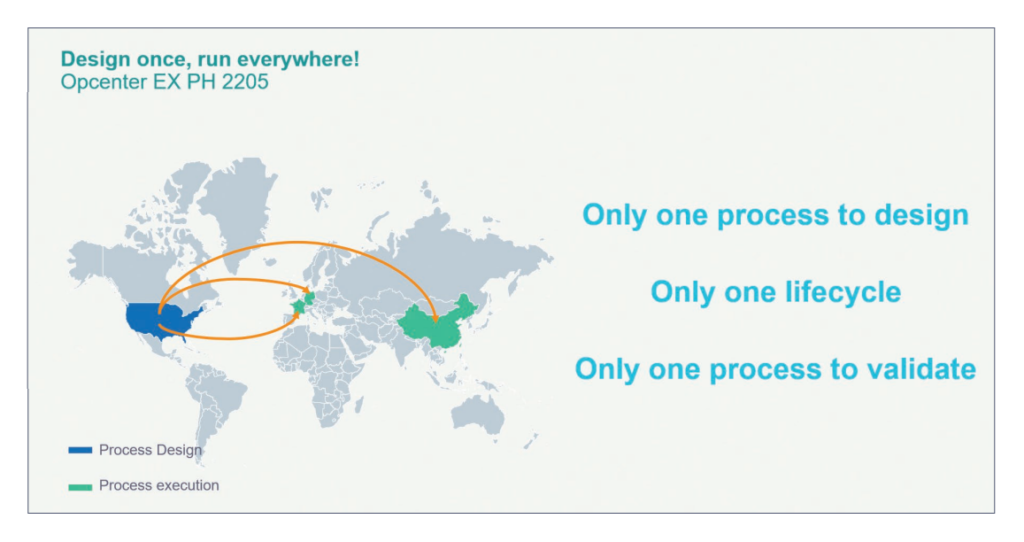
Achieving operational excellence
Using Opcenter EX PH 2205 enables manufacturers in the life sciences industries to achieve operational excellence, accelerate time-to-market, reduce compliance costs, and improve quality and productivity. Implementing Opcenter EX PH helps companies maximize the efficiency of all resources, providing user guidance, equipment allocation and operating procedures. It also allows companies to control and track every operation within production execution, whether human or automated. The system facilitates the review of the product batch record by exception, providing faster and more efficient product releases.
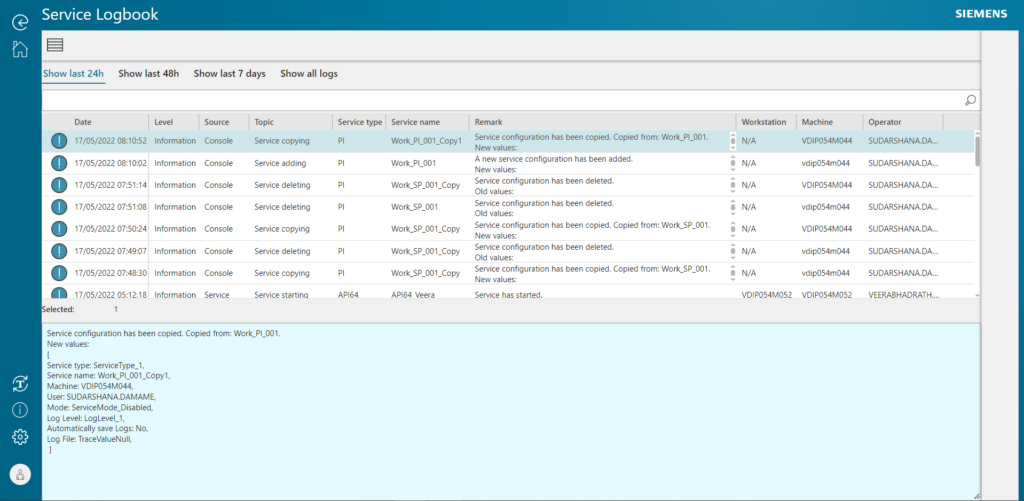
Some Opcenter Execution Pharma 2205 enhancements:
- Process Instruction multilanguage / translation support
- Work instruction enhancements
- HMI task list
- Design architecture and infrastructure design documentation
- System simplification with new service web console
- Openness of Weighing and Dispensing module
Benefits of Opcenter Execution Pharma 2205:
- Reduce paper usage until all processes can be handled electronically
- Reduce development effort and risk by integrating MES and DCS
- Standardize processes and facilitate implementation across sites
- Make batch-relevant information reviews faster and safer
Already using the Opcenter™ Execution Pharma? See what’s new in version 2205.
Want to learn more? With Opcenter Execution Pharma Siemens Digital Industries Software has developed a dedicated MES solution for the pharmaceutical industry that enables complete paperless manufacturing and full electronic batch recording. Opcenter Execution Pharma provides advanced features for designing, streamlining and managing production operations and processes – both manual and automated – via seamless integration between the MES, automation and enterprise resource planning (ERP) systems. Find out more about Opcenter Execution Pharma.


