What’s new in Opcenter Execution Medical Device and Diagnostics 8.9

Delivering enhanced technology that improves productivity, efficiency and quality
We are excited to announce that the latest version of Opcenter Execution Medical Device and Diagnostics 8.9 has been released!
Opcenter EX MDD 8.9, which is part of the Siemens Xcelerator portfolio, the comprehensive and integrated portfolio of software, hardware and services, delivers powerful enhancements to the application’s capabilities and security.
An enhanced representational state transfer application programming interface (RESTful API) facilitates streamlined interfacing and integration with the application. Improved data collection capabilities support greater control and enforcement of data point formats. A new scheduling route and route step provides greater flexibility when integrating with a scheduling application. Key security enhancements, such as a stronger cipher algorithm, provide an even higher level of security for the application. Finally, our expanded set of supported technologies offer access to the most up-to-date set of solutions to choose from.
Benefits of Opcenter Execution Medical Device and Diagnostics 8.9:
- Delivers an enhanced RESTful API for improved productivity, efficiency and quality
- Provides greater control and enforcement of a format for collecting data points
- Introduces improved flexibility for scheduling integration
- Delivers enhanced security and technology support
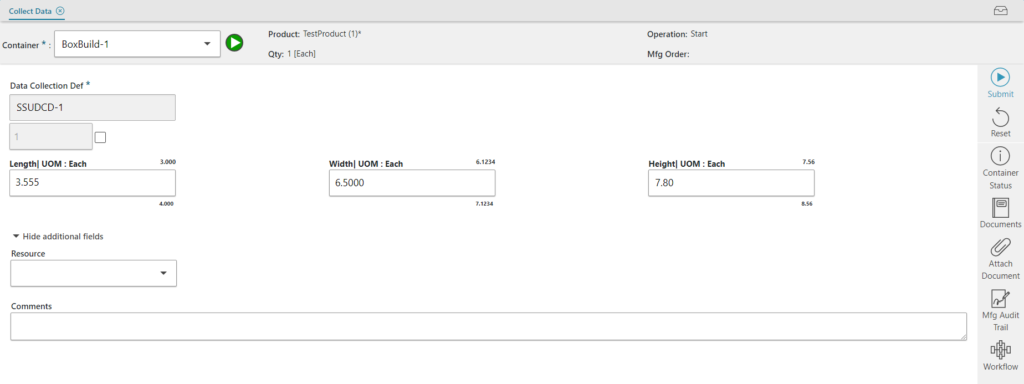
Already using the Opcenter Execution Medical Device and Diagnostics? See what’s new in version 8.9.
Want to learn more? Opcenter Execution Medical Device and Diagnostics serves as the foundation for top-tier global manufacturers and product innovators that want to enable rapid change, lean manufacturing, consistent quality output, rapid new product introduction (NPI) and higher profit margins. Find out more about Opcenter Execution Medical Device and Diagnostics.
eBOOK
Increasing complexity, regulatory requirements and supply chain issues led the medical device to be one of the most challenging industry for manufacturers to navigate.
Learn how to master your journey towards digital manufacturing now!
PODCAST
We invite you to hear our ultimate MD&D podcast episode from Dr. James B. Thompson, our Senior Director for Industry Strategy, about why Closed-loop Manufacturing is critical in the Medical industry in the new episode on The Voice of Smart Digital Manufacturing.
Tune in to learn more about the role digitalization is playing in helping companies solve problems and overcoming their challenges and spread the news!
Follow “The Voice of Smart Digital Manufacturing” on Spotify, Apple Podcast, Siemens ACast.
WEBINAR
Struggling with quality and medical device compliance? Learn how to overcome the ever-changing regulatory landscape and how closed-loop manufacturing drives innovation and cost reduction. Click here.

