The three pillars of operational excellence in medical device development

The medical device industry has historically addressed quality as a regulatory compliance requirement rather than a core business objective. However, the ability to rapidly adjust production rates, adapt layouts and procedures and flexibly respond to supply chain disruptions are business fundamentals.
Addressing quality as a core business objective is the root of operational excellence in medical device development. It can translate to greater success not only internally, but also with users and patients.
To achieve operational excellence, you need an intelligent, digital manufacturing foundation that connects your enterprise and creates a collaborative environment for accelerating communication and informed decision-making.
Operational excellence comprises three pillars: operational readiness, operational efficiency and operational quality. With each of these three pillars in place, you can:
- Respond faster to market needs by anticipating production and operational changes
- Increase the performance and agility of production operations
- Improve quality with flawless production data traceability
Let’s explore what these pillars mean.
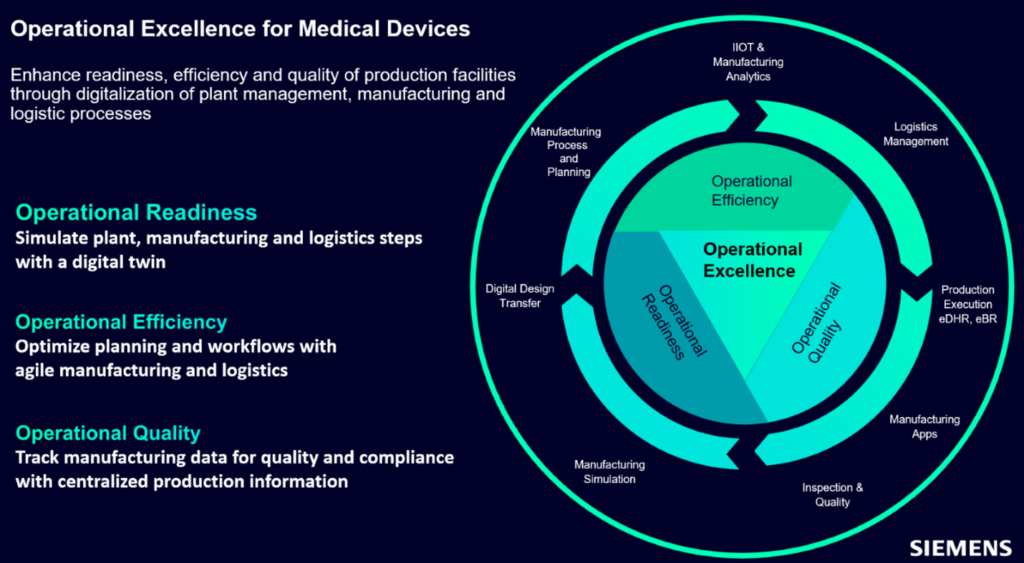
Pillar 1: Achieve operational readiness using a digital twin
Operational readiness involves digitally simulating parts, machines and plants. This helps manufacturers understand the expected performance in the physical world, anticipate issues before consuming resources and be ready to respond rapidly to changes.
Digital design transfer enables collaboration between research and design (R&D) and manufacturing teams. It also helps improve communicating data, changes and quality controls.
Plant layout and process simulation via digital twin provides an accurate way to view and personalize the extensive data operations generated by locating obstacles, testing solutions and validating new scenarios.
Additive manufacturing accommodates increasing patient demand for personalization and customization, allowing for more intricate manufacturing capabilities.
A digital machine shop connects the digital infrastructure using a digital thread, the data backbone that serves all functions while managing a centralized repository of product and process data.
Pillar 2: Achieve operational efficiency via planning and production workflows
Operational efficiency bridges the data and collaboration gap from design to manufacturing execution, implementing automatic planning and scheduling and optimizing production plans. Capabilities include:
- BOM management
- Process planning
- Process validation
- Product and process control
- Electronic work instructions
- Manufacturing information management
Pillar 3: Achieve operational quality by centralizing production information
Operational quality is the capacity to enforce processes and track production data in device history records or batch records, which ensures quality and compliance.
Centralizing production data enables you to track and collect all the information needed to validate mandatory market submissions. It also helps you execute quality processes such as LEAN management and the 5Ms of manufacturing (material, man, machine, measure and method).
A comprehensive manufacturing execution system (MES) error-proofs the manufacturing processes, creating a paperless manufacturing environment across batch and discrete operations.
How Siemens can help you achieve operational excellence
With Siemens Operational Excellence for medical devices integrated solutions, Medtech companies can leverage software that accelerates digital transformation. Our vision for tomorrow is an optimized network of digital enterprises sharing data and collaborating in the design, manufacture and deployment of products and processes.
Our Operational Excellence tools update your shop floor by ensuring that the correct resources are available, workers are properly trained, accurate test data is collected, and the right processes are followed. Integrating your systems helps improve collaboration within each location, between plants and with vendors.
Learn more about the three pillars of operational excellence
For a deeper dive into the three pillars operational excellence for medical device development, read our Using the three pillars of operational excellence white paper.
To talk to a Siemens Digital Industries Software representative, contact us here.


