PLM for Medical Devices: Deliver innovation at warp speed
The medical device industry is constantly faced with a variety of challenges. Market pressures are in the back of mind for medical device manufacturers as prices increase. As products become more connected to the Internet and other devices, manufacturers must find a way to keep costs at bay but still provide innovative and smart products to patients. And as always, regulatory standards continuously change from region to region, and teams must navigate the murky waters of compliance.
All of these factors drive companies to look to invest in product lifecycle management (PLM) tools.
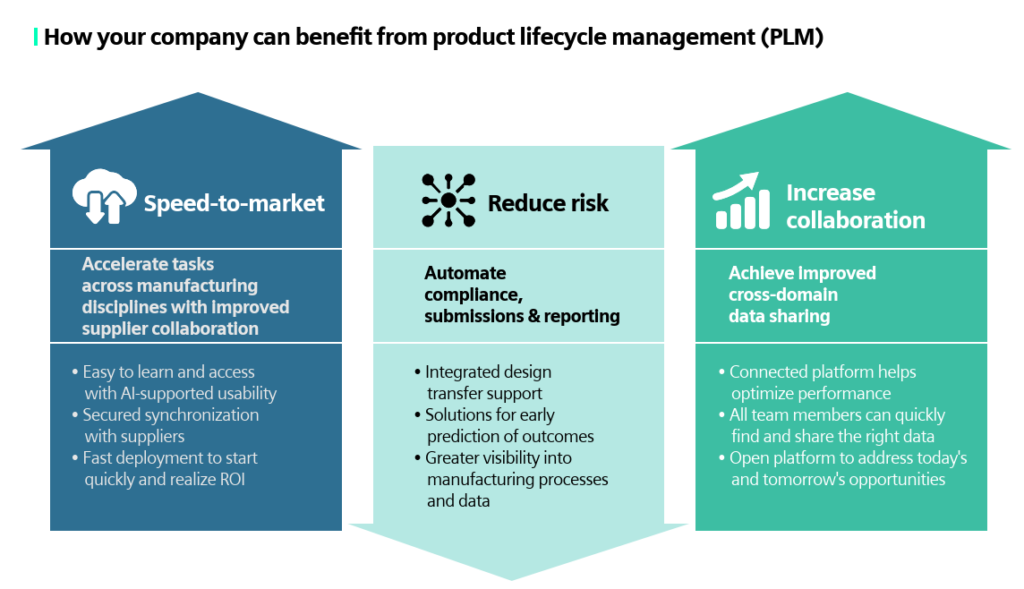
How can you benefit from PLM?
- Speed to market: Accelerate tasks across manufacturing disciplines with improved supplier collaboration.
- Reduce risk: Automate compliance, submissions and reporting.
- Increase collaboration: Achieve improved cross-domain data sharing.
Why implement a SaaS solution?
With Siemens PLM for Medical Devices, our solution is easy to learn and access with AI-supported usability, comes with secured synchronization with suppliers and has fast deployment to start quality and get a return on your investments. Our SaaS solution has integrated design transfer support and can provide greater visibility into the manufacturing processes and data. The connected platform helps to optimize your devices’ performances and allows your team to quickly find and share the right data.
The new SaaS PLM for Medical Devices can:
- Increase reliability
- Reduce overhead
- Lower costs
- Allow global access
To discover the efficiencies of PLM for Medical Devices and overcome today’s challenges, click here to read the newly published infographic.
To find more resources about PLM for Medical Devices, click here to learn more about the cloud-based product development system.
Interested in more blogs about the medical device industry and how Siemens can help, click here.


