How to improve medical device risk management

As medical devices become more complex and powerful, the regulatory environment does so as well. Mitigating risk in a medical device is top priority when it comes to patient safety. The concept of medical device risk management may seem simple – remove any risk to using a device and it is safe. However, when it comes to compliance, things in the medical device industry are never as simple as they seem. All products must comply with certain specifications to be used for their intended purpose, which is done through verification and validation (V&V). Risk management complexity is caused by many factors and variables, and tracking those components requires a powerful solution.
Challenges of medical device risk management
Difficulty in risk management comes from using old technology to track a complex environment. Static written documents and spreadsheets are outdated. Developers face the challenge of implementing an effective risk management system. Medical device risk management must be based on living design elements that can easily be shared from a central repository. Without automation, design intent can be lost across workflows, product changes and requirements updates.
Other challenges that developers face include:
- Incorporating key standards, such as ISO 14971:2009, ISO TIR 24971:2013 and EN ISO 14971:2012
- Covering a full range of associations from risk analysis to post-market surveillance with a complete risk management solution
- Tracking all of the interdependencies of risk analysis, control, reporting and surveillance
Achieving comprehensive traceability
Traceability is the foundation of medical device risk management. Teams must be able to trace interrelated work items and their relationships to design and regulatory requirements. Below is a figure illustrating the building blocks of traceability seen in our medical device traceability table. This figure shows elements that provide the basis for developing the design V&V test plan and risk management framework.
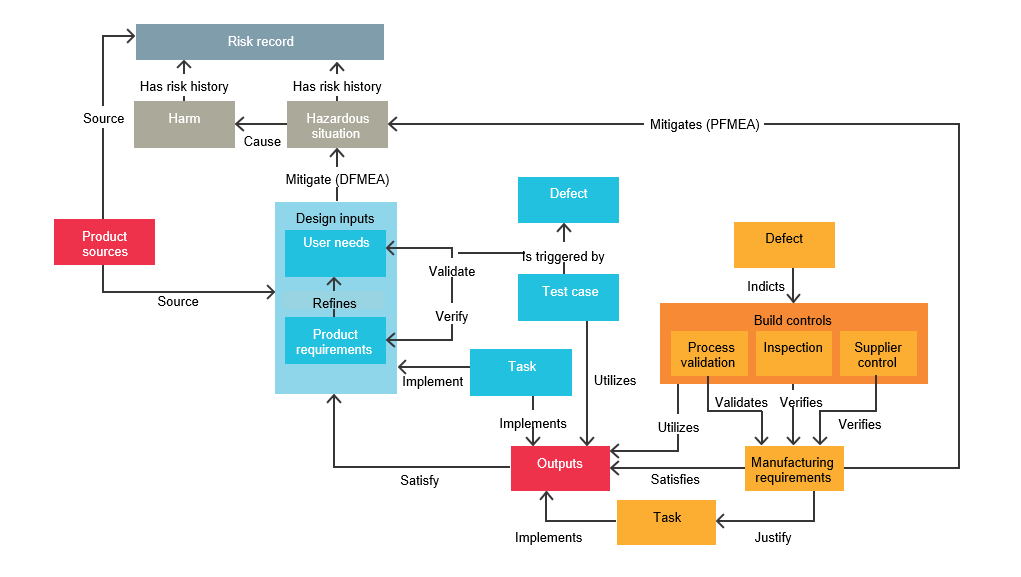
The complexity of the interrelations shown in the figure above cannot be tracked easily by spreadsheets. An efficient solution for managing product requirements should make it easy to associate user needs with product and software requirements and relevant testing. A robust requirements management solution supports workflows and traceability throughout the design, manufacturing and risk management relationships typical in the medical device product development process.
Some best practices for organizing components for traceability include:
- Organize data by how it will be reviewed
- Use common terminology for data fields
- Minimize linking complexity
Integrating a risk management data model
A well-designed, comprehensive risk management tool should be able to support a risk management data model that ensures consistent use of definitions, work items and workflows. This will allow teams to identify and mitigate harms and hazards more easily. A risk management data model brings precision to a company’s operations. Teams can set definitions based upon the design or regulatory requirements and then enforce consistency of use to enhance data quality.
Creating a risk management data model using our solution for medical devices gives companies the ability to develop enforceable workflows so that all team members use the same defined workflows. This provides a unifying framework to ensure that tasks can be completed without leaving anything out.
Learn more about our solution for medical devices.
Interested in more medical device blogs? Read more here.


