Strengthen medical device supply chains with a shift-left approach

Healthcare innovation is advancing rapidly – from AI-supported diagnostics and connected monitoring to next-generation robotic surgery and wearable therapeutic devices. Yet behind these breakthroughs lies a growing challenge: the global supply chains that support medical device manufacturing are more exposed than ever to disruption.
From geopolitical volatility to resource scarcity and increasing regulatory complexity, the supply chain risks facing biomedical devices are not just operational, they’re existential. As medical device companies work to deliver smarter, faster and more sustainable solutions, a new mindset is required – one that embeds supply chain resilience directly into medical design and development processes.
A new Siemens infographic, “Vulnerable supply chains in the medical device industry” explores these issues and shows why the traditional downstream focus on supply chains is no longer sufficient.
Global sourcing creates local vulnerabilities
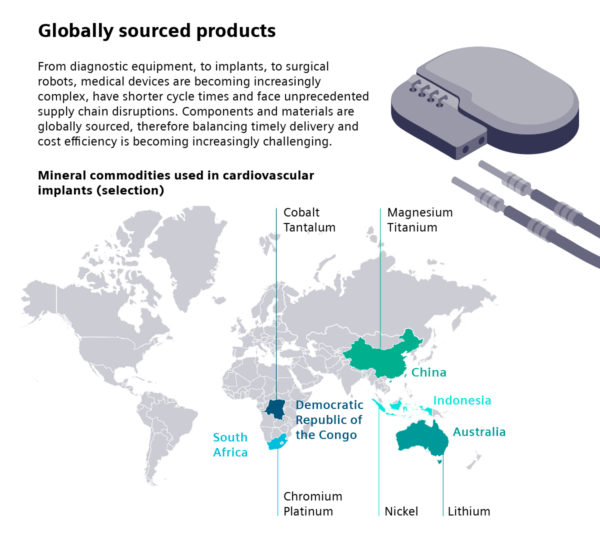
Medical devices depend on a vast, interconnected network of raw materials and components, many of them sourced globally. Critical materials like tantalum, lithium and rare earths power everything from implantable devices to surgical robotics. While these commodities enable innovation, they also introduce risk.
As shown in the infographic, medical devices are the third most vulnerable sector to global trade disputes, after semiconductors and communications technology. This level of exposure can lead to unpredictable shortages, higher costs and production delays. It’s especially damaging when they affect medical device prototyping and regulatory approval timelines.
Unplanned downtime: a persistent threat
Disruptions aren’t hypothetical, they’re routine. Across industries, 85% of organizations report regular unplanned downtimes, many linked directly to supply chain issues. In the medical device industry, even minor delays can ripple through the system, slowing innovation and impacting patient care.
Worryingly, 44% of respondents report that these downtimes occur every two months or even more frequently, emphasizing the need for proactive, not reactive, strategies – especially in biomedical device manufacturing, where lead times, compliance and precision are critical.
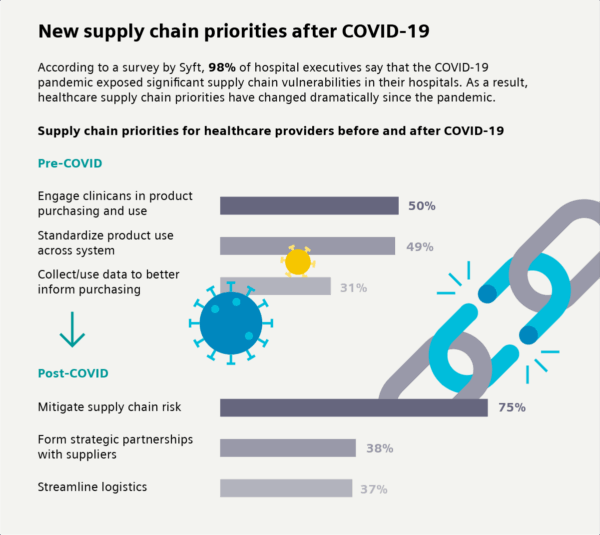
A shift in MedTech priorities post-COVID
The COVID-19 pandemic revealed the fragility of healthcare supply chains. A Syft study found that 98% of hospital executives now rank supply chain visibility and resilience among their top concerns. Where once the focus lay in clinical engagement and procurement standardization, today’s healthcare systems are prioritizing:
- Supply chain risk mitigation
- Strategic supplier partnerships
- End-to-end logistics integration
This strategic pivot places new expectations on medical device software and hardware teams. Design and development must now account for sourcing, transportation and regulatory logistics from day one.
Resilience through digital transformation
To meet these challenges, medical device manufacturers are increasingly turning to digital transformation. By integrating logistics and sourcing data into early-stage medical design and prototyping, companies can shift supply chain considerations upstream.
This “shift-left” approach explored in the Siemens white paper ”The Shift-Left Logistics Imperative” enables product development teams to analyze supplier risk, material availability, cost implications and even carbon footprint as part of the design phase. The result is a product that is not only functional but also manufacturable and deliverable – at scale and on time.
Smart software, stronger supply chains
Advanced medical device software is not only vital for powering today’s smart biomedical devices, it’s equally essential for optimizing the complex supply chain systems that support their development and delivery. With Siemens’ Digital Logistics portfolio, including cloud-based solutions such as the supply chain digital twin and supply control tower, medical device manufacturers can create more agile, transparent and resilient operations.
These solutions enable companies to:
- Simulate supply chain scenarios in real time
- Predict and prevent potential bottlenecks before they impact production
- Streamline warehouse and distribution strategies
- Maintain full traceability and meet stringent compliance requirements
When integrated early in the medical device development lifecycle, these capabilities help align engineering, logistics and regulatory priorities, making innovation not only achievable, but scalable and sustainable.
By connecting every function through a unified digital thread, Siemens enables medical device companies to transform operational risk into resilience – and turn disruption into a lasting competitive edge.
Learn more about medical device supply chains
Explore our infographic “Vulnerable supply chains in the medical device industry”, to see how shifts in design, sourcing and logistics are reshaping the future of biomedical devices.
For even more details on our shift-left approach, download the white paper ”The Shift-Left Logistics Imperative.”


