Why MES for emerging Medical Device manufacturers
There’s a common belief that manufacturers need to have attained a certain scale before considering investing in technology like MES, but an increasing trend among emerging medical-device companies is challenging this.
Emerging companies face unique challenges. Those who are at or near FDA approval are at the point of commercialization, and this means significant change is at hand. In the past, they had to address very small volume, but as products move toward regulatory approval, growth—and growth in volume—comes rapidly and significantly. Volumes can grow by a factor of 5x or 10x in a matter of months.
For emerging medical-device manufacturers, this is the perfect storm; one that means they have to invest in infrastructure. Increasingly they are turning to MES to help manage growth and change, which if not managed properly can result in product-quality issues—a death knell for any nascent concern.
Three principal benefits are driving emerging medical-device manufacturers to invest in MES:
- The high-value role of information
- Error proofing
- Achieving paperless manufacturing
 For these emerging concerns, the high-value role of MES is principally seen in basic work-in-process tracking and genealogy because, during this stage of development, they need to stabilize their process, make it repeatable, and achieve good yields. They need information to do this (e.g., for key tasks like root-cause analysis), and MES delivers it.
For these emerging concerns, the high-value role of MES is principally seen in basic work-in-process tracking and genealogy because, during this stage of development, they need to stabilize their process, make it repeatable, and achieve good yields. They need information to do this (e.g., for key tasks like root-cause analysis), and MES delivers it.
 Another key area is error proofing. As companies go from 10 employees to 50 or 100, managing and providing oversight becomes a much more daunting task. The task is complicated further by the fact that these are new employees. By automating process compliance, MES helps ensure consistent quality with “right first time” execution. Beyond the obvious demands of regulatory compliance and competing with much larger companies—and trying to establish their own brand in the midst of a sea of established brands—quality is not an option for emerging companies. They need it to be a positive differentiator.
Another key area is error proofing. As companies go from 10 employees to 50 or 100, managing and providing oversight becomes a much more daunting task. The task is complicated further by the fact that these are new employees. By automating process compliance, MES helps ensure consistent quality with “right first time” execution. Beyond the obvious demands of regulatory compliance and competing with much larger companies—and trying to establish their own brand in the midst of a sea of established brands—quality is not an option for emerging companies. They need it to be a positive differentiator.
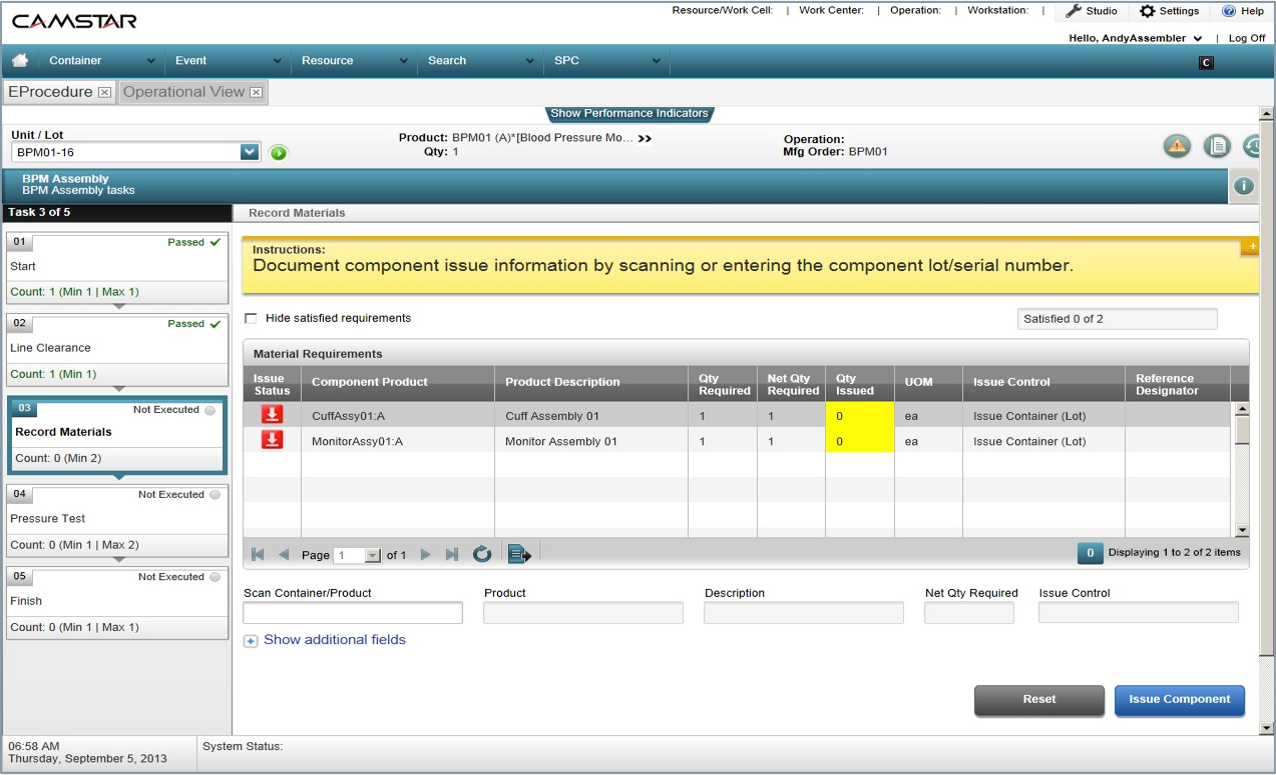
 Finally, MES helps eliminate the inefficiencies of paper-based systems that emerging medical-device concerns typically have relied on. In paperless manufacturing, electronic records replace paper-based, manual ones. There are no “paper travelers” around the facility or across facilities, and paperless systems are typically integrated into higher-level systems like ERP. Operators in paperless manufacturing facilities are presented with production tasks in properly ordered sequences (e.g., by priority, due date, or customer significance). Operators are given visual cues, such as key performance indicators, at their work cells to help enforce and speed proper action. Drawings, graphics, and standard operating procedures are viewed online, ensuring that workers are working from their most current version. Paperless systems not only prevent errors—they provide real-time alerts when issues arise.
Finally, MES helps eliminate the inefficiencies of paper-based systems that emerging medical-device concerns typically have relied on. In paperless manufacturing, electronic records replace paper-based, manual ones. There are no “paper travelers” around the facility or across facilities, and paperless systems are typically integrated into higher-level systems like ERP. Operators in paperless manufacturing facilities are presented with production tasks in properly ordered sequences (e.g., by priority, due date, or customer significance). Operators are given visual cues, such as key performance indicators, at their work cells to help enforce and speed proper action. Drawings, graphics, and standard operating procedures are viewed online, ensuring that workers are working from their most current version. Paperless systems not only prevent errors—they provide real-time alerts when issues arise.
Bottom line: emerging medical-device manufacturers leverage MES in virtually the same way as their larger competitors leverage them. The challenges they face are the same as the “big boys;” they’re simply at a different point in the corporate evolutionary process. Increasingly, they see the value of incorporating MES at this earlier point, to speed their transition from emerging to small to mid-sized or large. After all, that is the goal, and MES is an undeniable—and increasingly recognized—asset as they move toward it.