NOW AVAILABLE: SIMATIC IT R&D Suite 7.3
We are excited to announce that Siemens SIMATIC IT R&D Suite 7.3 is now available! SIMATIC IT R&D Suite V7.3 is the fourth version released on the “next generation” platform for SIMATIC IT R&D Suite, which is the new platform that will embed all functionality from the Classic product portfolio: SIMATIC IT Unilab, SIMATIC IT Interspec, and R&D Libraries (ELN/FWB). It represents a major technologic advancement, providing lightweight client applications to the user with the latest web technology and HTML5.
Version 7.3 offers new capabilities in Product Specification Management and P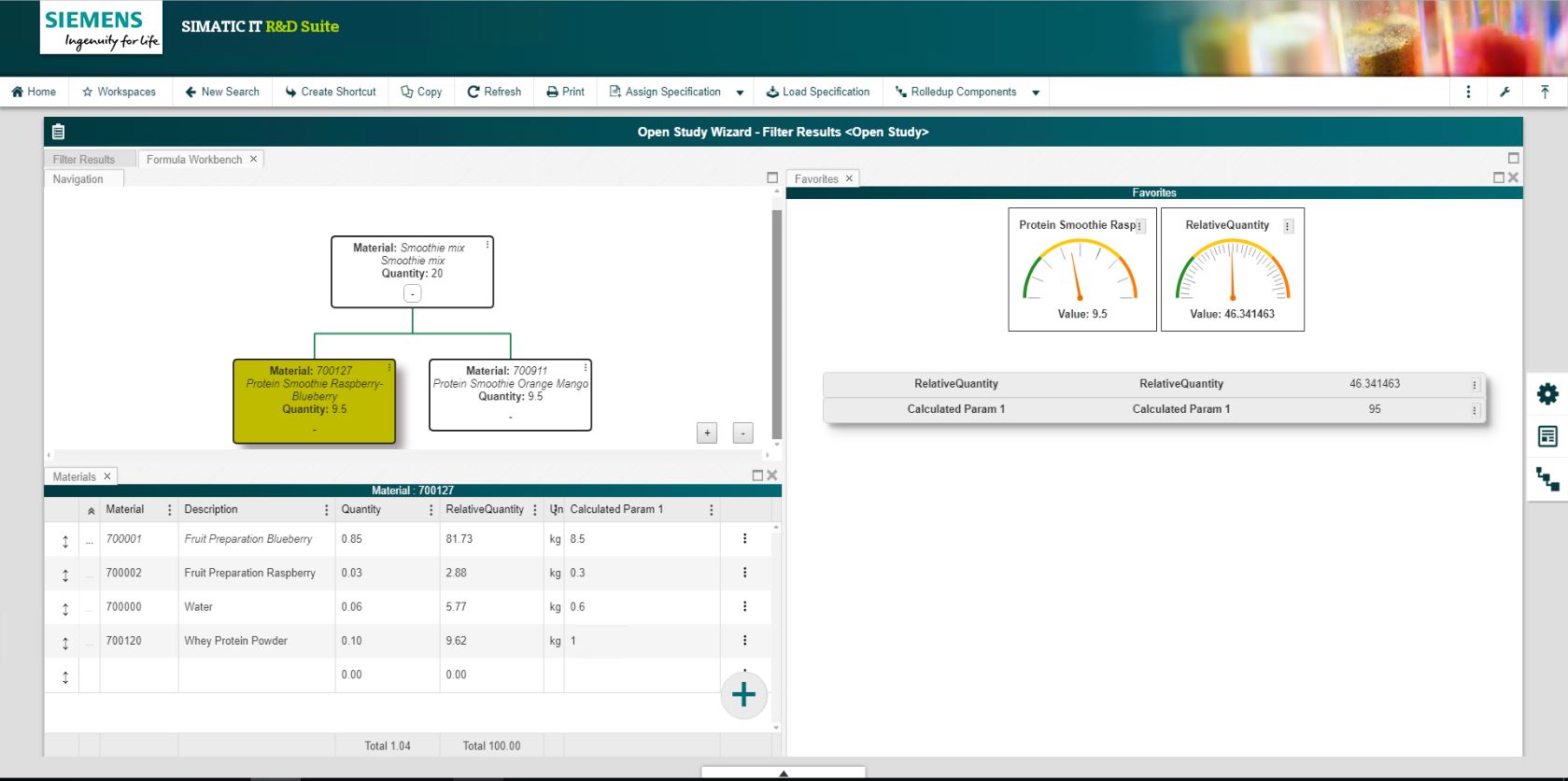 roduct Formulation, including:
roduct Formulation, including:
- Specification Management (BoM Explosion, Nutritional Declaration, Support of Attached Specifications)
- Formula Workbench
- Context-based Result Validation
- Fully Configurable Launchpad
- SWAC Integration in Unified Architecture
- Flex LM Licensing
- Plus many other usability improvements
SIMATIC IT R&D Suite manages the entire R&D process in a structured, yet flexible manner to enable fast and successful new product development. It shares a common system platform with production processes to facilitate the transfer of new product designs into production, optimizing production cost and quality.
Find out more about the latest version of SIMATIC IT R&D Suite.
Comments
Leave a Reply
You must be logged in to post a comment.



What are the options to integrate Formula WorkBench with 3rd Party Systems for regulatory compliance and analytical requirements ?
Dear Madhukar,
Please note that SIMATIC IT R&D Suite has a build-in capability for regulatory assessments – for example allergens, free-from, food and health claims, nutritional profiles, …
Reference data and legal thresholds for a specific country can be configured in the R&D Suite (often also an “interpretation” of the company).
For standard regulatory compliance reference data covering 212 countries – with live update service available – we are working with our partner Decernis
For an illustration how this works, please consult :
https://sales.industrysoftware.automation.siemens.com/more_info/66016/Regulatory-Compliance-Use-Case-SIT-RandD-Suite-gComply-integration-Presentation